Chapter 1 Introduction to Photochemistry & Photophysics
Let us open with some context on this course.
Photochemistry is, by definition from IUPAC, ‘the branch of chemistry concerned with the chemical effects of light’1. However, in this course we are going to focus on and examine some of the fundamental photophysics of system before finally introducing chemical reactions involving light. Photophysics is closely related to what you have previously studied in your first and second years, in that it is a study of the processes of absorption and emission of light and the kinetics of these processes. We will also draw upon your previous studies of quantum mechanics as wavefunctions are vital in our understanding of photophysical processes.
Light is the very reason why there is life on earth, the outstanding beauty of processes such as photosynthesis or vision speak to the power of this branch of chemistry. Photochemistry offers a way for us to ‘cheat’ traditional chemical reactions, in that we no longer need thermal energy to get over an activation barrier, instead we use the energy of a photon. It is only by understanding how we can best utilise the bounteous resource of the sun’s light that we as a species can hope to to have a long and fruitful future on this planet.
It is just over 100 years since quantum theory revolutionised our understanding of matter and light, 125 years since the discovery of the electron, but in that time we have achieved wonderful things, things that have revolutionised the way we live. From lasers in cd and blue ray players to their use in scanning bar codes, glow sticks (fun at festivals, essential in emergencies), or more prosaic uses such as sun creams, self cleaning windows or display technology (where would we be without the phones in our pockets?). In this course you will learn about a range of photophysical and photochemical processes that underpin our modern lives and also learn about some of the current challenges and interests in the field.
Spectroscopy
- techniques
Photochemistry
- reactions
- molecular structure
Photophysics
- kinetics
- thermodynamics
- quantum mechanics
Photophysical processes
*transitions which convert between excited states or between an excited state and a ground state of a molecule
Photochemical processes
*reactions or rearrangements which occur as a consequence of excitation from the ground state
1.1 Assumed background knowledge
The course relies heavily on first and second year kinetics concepts as well as building on the quantum mechanics from second year and some of the spectroscopy you did in first year. Whilst I have done my best to ensure that there is a minimum of non-physical chemistry content required to get the most out of this course if I want to talk about molecules some very basic knowledge from both organic and inorganic chemistry, much of which will at least have been mentioned at ‘A’-level or IB.
I will talk a lot about \(\sigma\) and \(\pi\) bonding, but this has been covered in quantum mechanics, a double bond has one \(\sigma\) and one \(\pi\) bond. I will also talk about shape and structure, a carbon with only \(\sigma\) bonds is 3 dimensional and tetrahedral, a carbon with 3 \(\sigma\) and 1 \(\pi\) bond is trigonal planar, and one with 2 \(\sigma\) and 2 \(\pi\) bonds is linear.
However we need to think about the fact that a molecular orbital exists over a hole molecule. If we reduce bond order overall we change the shape of the molecule. This will be discussed in the absorption (Chapter 2) section of this course.
If you need more help on structure, bonding or metal complexes Chemistry3 is an excellent book to start with, or please ask for help.
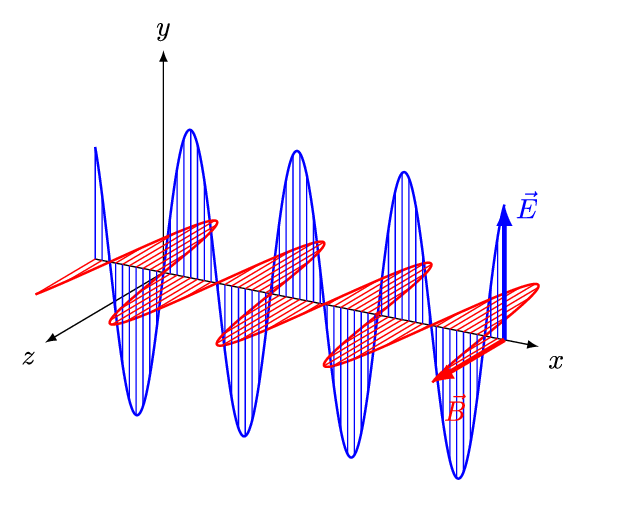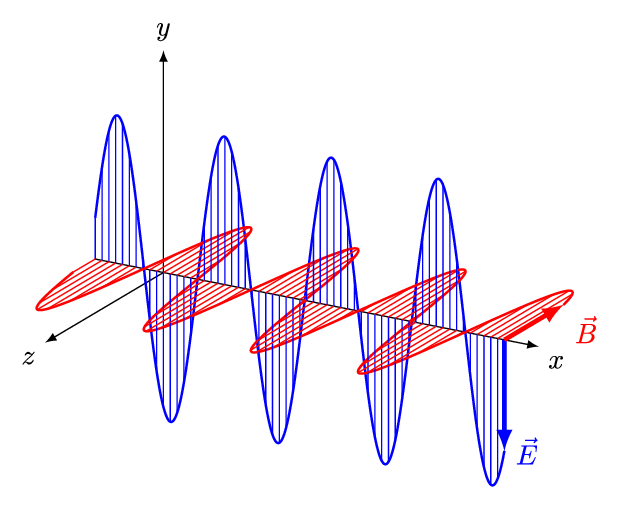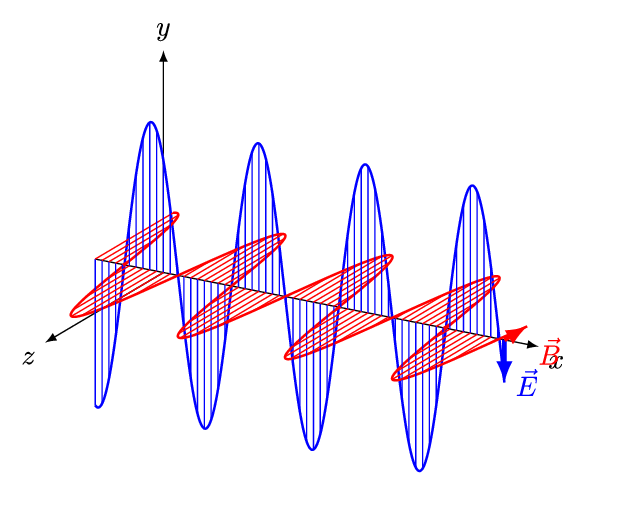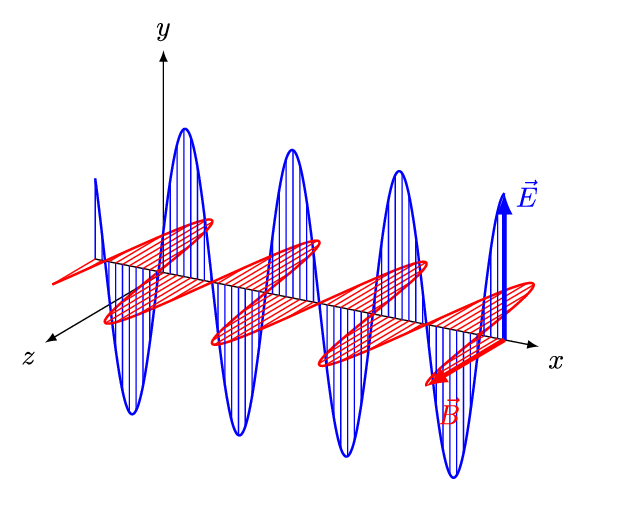
1.2 Light
As you’ve already learnt light exhibits properties that has both a wave like and particle like nature, and our understanding of light was one of the fundamental pieces that lead towards the quantum theory of the atom. Newton had shown that light from the sun is comprised of a spectrum of colours, we now know that that spectrum goes beyond what we can see with our eyes.
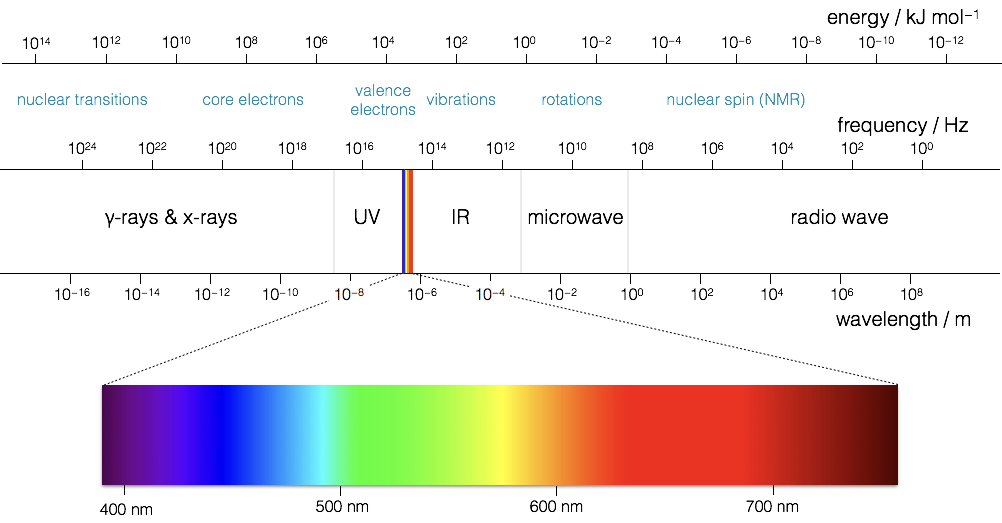
Figure 1.2: The electromagnetic spectrum of light, frequency and energy are equivalent, so high frequency waves have high energy photons
In your studies you have already seen how large amounts of the electromagnetic spectrum is used for different spectroscopic techniques, making use of the quantised transitions within molecules. For example, infra-red light has the same energy as the vibrational transitions within molecules. In photochemistry, we are specifically interested in the visible and near UV part of the EM spectrum as photons with this energy promote transitions for the valance electrons in the molecules we are interested in.
It is important never to forget the wave particle duality of light, however for much of photochemistry and photophysics it is perhaps simpler to consider the light as a stream of incident photons, whilst there are classical (wave) models to thing of processes like absorption and emission this set of notes will usually defer to an entirely particle model of absportion or emission of a photon.
1.3 Energy levels
One of the consequences of quantum theory is that energy levels within atoms and molecules are discrete (figure 1.3). Atoms only have electronic and translational energy levels, whereas molecules have electronic, vibrational, rotational and translational energy levels (in decreasing order of energy steps).
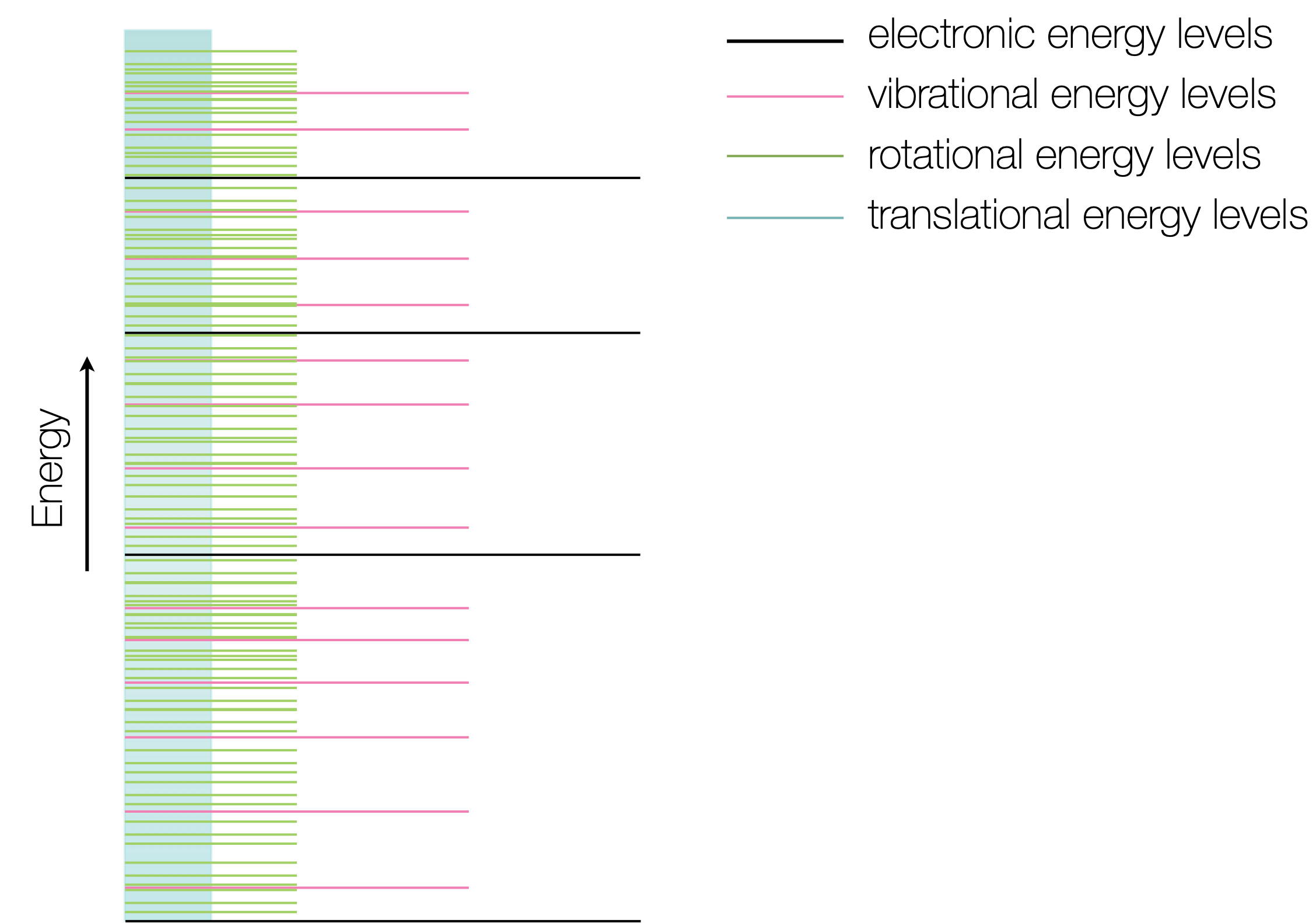
Figure 1.3: Molecules have a number of different energy levels over which the internal energy of a molecule is distributed. Translational energy levels are essentially a continuum, however rotational vibrational and electronic levels all have measurable transitions.
As you have seen in figure 1.2 these transitions between these energy levels correspond to different parts of the electromagnetic spectrum, however the presence of translational, rotational and vibrational energy levels also has an effect on the transitions between electronic energy levels.
Translations are movement of the whole molecule along the xy&z cartesian coordinates, rotations can be either the whole molecule rotating (along the xy&z axis) or else internal rotations within the molecule. Vibrations include both stretching and bending motions within a molecule.
IUPAC Goldbook, http://goldbook.iupac.org/terms/view/P04588 (accessed July 2020).↩︎